Polyatomic Ions: Conquering Chemistry's Tiny Teams
Ready to master polyatomic ions? These negatively or positively charged groups of atoms are fundamental to chemistry. This guide blends explanations, examples, and quizzes for effective learning. Did you know that understanding polyatomic ions is key to unlocking a deeper understanding of various chemical processes, from everyday reactions to advanced research? Test your knowledge with this polyatomic ions quiz.
Understanding Polyatomic Ions: It's Easier Than You Think!
Imagine a tightly bound group of atoms acting as a single unit with an overall charge – that's a polyatomic ion! They're not just random collections; specific combinations of atoms forming charged units crucial in chemical reactions. From baking soda (sodium bicarbonate) to compounds in your body, they're everywhere! Mastering them significantly enhances your understanding of chemical reactions and compound formation.
Common Polyatomic Ions: Meet the Usual Suspects
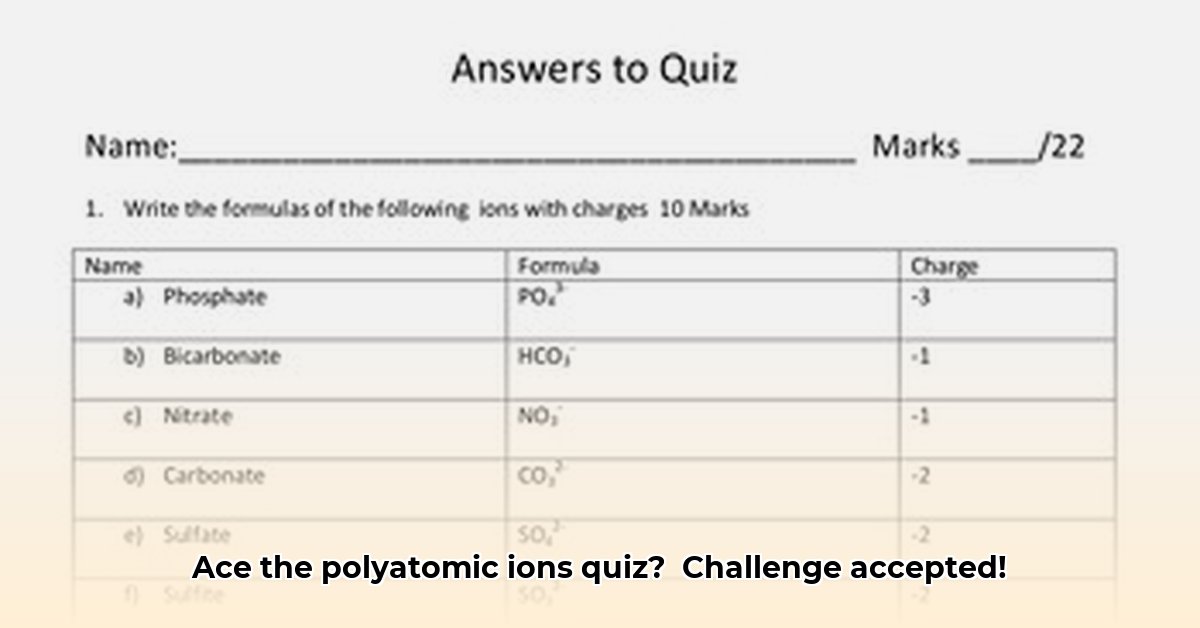
Let's familiarize ourselves with frequently encountered polyatomic ions. Learning their names and formulas is the first step. Think of it as expanding your chemistry vocabulary; repetition will make it second nature.
| Ion Name | Formula | Charge | Commonly Found In... |
|---|---|---|---|
| Sulfate | SO₄²⁻ | -2 | Epsom salts, fertilizers |
| Nitrate | NO₃⁻ | -1 | Fertilizers, preservatives |
| Phosphate | PO₄³⁻ | -3 | Fertilizers, DNA/RNA |
| Carbonate | CO₃²⁻ | -2 | Limestone, baking soda |
| Ammonium | NH₄⁺ | +1 | Fertilizers, cleaning products |
| Hydroxide | OH⁻ | -1 | Bases, many metal hydroxides |
| Acetate | CH₃COO⁻ | -1 | Vinegar, many organic compounds |
Note that some ions carry a negative charge (anions) and others a positive charge (cations). This charge is vital for their interactions and compound formation. How many of these ions do you already recognize from your everyday life?
Quick Quiz 1: Test Your Knowledge!
Let's check your understanding. Review the table before answering.
- What's the formula for the sulfate ion?
- What is the charge on a nitrate ion?
- What is the name of the polyatomic ion with the formula PO₄³⁻?
- Which is a cation (positive ion): NH₄⁺ or OH⁻?
Answers: 1. SO₄²⁻, 2. -1, 3. Phosphate, 4. NH₄⁺
Applying Your Skills: Level Up!
Knowing names and formulas is crucial, but understanding their behavior in chemical reactions elevates your mastery.
1. Balancing Equations: Treat polyatomic ions as single units when balancing equations; they remain intact.
2. Predicting Products: Use your knowledge to predict compound formation when ions react. It's like solving a chemical puzzle!
3. Naming Compounds: The rules for naming compounds containing polyatomic ions differ slightly from those without. Practice this crucial skill.
Quick Quiz 2: A More Challenging Question
What are the products of the reaction between sodium hydroxide (NaOH) and sulfuric acid (H₂SO₄)? (Consider the interaction between positive and negative ions.)
Answer: Water (H₂O) and sodium sulfate (Na₂SO₄).
Mastering Polyatomic Ions: Tips for Success
Consistent practice is key. Here's how to excel:
- Flashcards: Create flashcards with ion names on one side and formulas/charges on the other.
- Online Quizzes: Many websites offer quizzes with immediate feedback.
- Practice Problems: Work through textbook or online problems for reinforcement.
- Focus on Understanding: Although memorization is necessary, grasp the underlying principles for better retention. Understanding the structure and bonding within the polyatomic ion is key to understanding its behavior.
Remember, mastering polyatomic ions takes time and practice. Don't hesitate to seek help when needed. You've got this!
Advanced Applications: Polyatomic Ions in Research
From Basics to Research: Demystifying Polyatomic Ions
Polyatomic ions are fundamental building blocks in countless chemical reactions. Think of them as specialized LEGO bricks—each with unique properties that combine to create complex structures. Understanding them opens doors to advanced chemistry.
First, let's reinforce the basics. Polyatomic ions are covalently bonded atom groups carrying a net charge. This charge dictates how they interact with other ions, influencing the entire chemical reaction.
Mastering Nomenclature: The Chemistry Language
Accurate nomenclature (naming) is essential for clear communication. Can you readily name ions like sulfate (SO₄²⁻) and phosphate (PO₄³⁻)? Practice using flashcards, quizzes, or mnemonics until you're fluent in this chemical language.
Dr. Anya Sharma, Professor of Chemistry at MIT, emphasizes, "A strong grasp of polyatomic ion nomenclature is the cornerstone of advanced chemistry research. Without it, accurate representation of chemical events is impossible."
Beyond Memorization: Understanding Reactivity
Understanding why ions behave as they do is crucial. Analyze the structure and bonding within the ion. How does electron distribution impact charge and reactivity? This deeper insight gives you a significant advantage in advanced research.
Real-World Applications: Expanding Your Knowledge
The applications of polyatomic ion knowledge are vast:
- Spectroscopy: Polyatomic ions have unique spectral signatures, aiding identification and quantification in samples.
- Redox Reactions: Their electron transfer properties are crucial for designing and interpreting redox reactions.
- Materials Science: They are vital in designing materials with specific properties (e.g., conducting polymers, superconductors).
- Biochemistry: Many biomolecules, such as nucleotides and amino acids, contain polyatomic ions.
- Environmental Chemistry: Their environmental behavior and impact on water and soil chemistry are critical for remediation strategies.
Advanced Techniques and Analysis
Advanced chemistry research requires more than ion recognition:
- Quantitative analysis: Precisely calculating concentrations and yields.
- Data interpretation: Extracting key information from experiments.
- Problem-solving: Developing effective strategies for complex research questions.
Mastering these skills empowers you to tackle challenging projects and make significant contributions to the field of chemistry.